Mutagenic impurities can be expected in any drug product; however, this is not a concern providing they are present below the safe limit. The objective of a carcinogenicity study is to identify compounds that can cause tumours in animals, which is imperative to understanding the cancer risk posed to humans.
ICH M7 in practice
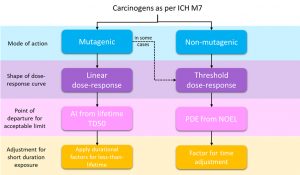
The ICH M7 guideline is designed to establish the carcinogenic risk posed by mutagenic impurities, considering both safety and quality risk management. If a compound reacts with DNA it may lead to a mutation, which is the first step in the development of cancer. This can be a risk even for low level impurities.
The guideline also states that some compounds may not be mutagenic but have the potential to cause cancer by other mechanisms. However, as it is not expected that these compounds would be a concern when present at low levels, they are out of the scope of ICH M7.
There are two known ways to define the safe limit for carcinogens, depending on the mode of action and dose response, as shown in the image below.
Check out this short video in which Principal Application Scientist, Fernanda Waechter, illustrates in greater detail how to calculate the safe limit for carcinogens.
Lhasa or Gold TD50: Which values to use?
As Fernanda explains, TD50 values are an important aspect of carcinogenicity assessment. TD50 values indicate the lifetime dose at which tumours would be observed in 50% of animals, which would have remained tumour free at 0 dose – but did you know there are two TD50 values in the Lhasa Carcinogenicity database (LCDB)?
The LCDB was founded in 2016 on the now retired Carcinogenic Potency Database (CPDB), created by Lois Gold and her team. With a redesigned interface, it is a freely available resource containing the results of animal cancer tests.
We calculated our own Lhasa TD50 values based on those provided by Lois Gold and her team, producing a transparent methodology for calculating TD50 values from experimental data, in a manner consistent with the CPDB. Displaying both TD50 values in the LCDB has enabled validation of the calculation method by comparison with the original CPDB values. As we expand the LCDB data set, we are able to calculate TD50 values for the newly added data.
Through its ongoing availability, the LCDB continues to support users’ carcinogenicity risk assessments by enhancing decisioning making and improving productivity.
Please get in touch If you would like to learn more about the LCDB and how it can support your toxicity assessments.
Last Updated on January 25, 2024 by lhasalimited



