By 2025, 36% of world data will be healthcare data. (Yes, thirty-six percent!). Considering this statistic, now more than ever, it is important that we are working to ensure existing data is utilised to make better safety assessment decisions more quickly – whilst also relying less on animal testing.
Within many sectors of the life sciences industry, animal testing is still considered a primary option for toxicity testing, and as a result is used heavily – especially within the pharmaceutical sector (In 2021, the Home Office found that in Great Britain 43% of animal testing for regulatory purposes was for medicinal products for human use and 30% for veterinary products – see page 21 of the report ‘Annual Statistics of Scientific Procedures on Living Animals Great Britain 2021’).
However, the many limitations of this model are now well understood and non-animal new approach methodologies (NAMs) present an ideal opportunity to improve on this current approach.
There are now a range of individual non-animal approaches which can provide human relevant results for safety assessment however, the challenge comes in how this evidence can be combined to replace animal testing. This is where Adverse Outcome Pathways (AOPs)* offer a unique solution, AOPs allow individual pieces of evidence to be linked in a meaningful way, even more so when visualised within an AOP network**. Linking individual pieces of evidence in this way improves mode of action understanding and allows the coverage of endpoint-relevant biological mechanisms to be better assessed, leading to more informed safety assessment decisions.
Several prominent bodies advocate this knowledge structure to contextualise evidence including the OECD, EPA and European Commission. However, using AOPs to contextualise NAMs and to make complex endpoint safety assessment decisions, instead of animal testing, is not yet broadly accepted by regulators in the majority of safety assessment settings. As a result, we are at the forefront of driving this science, particularly within the pharmaceutical industry.
For the last five years, Lhasa scientists have been creating, curating and peer reviewing AOPs to build a coherent AOP knowledge network – focusing on the carcinogenicity (genotoxic and non-genotoxic mechanisms) and developmental and reproductive toxicity (DART) chemical space initially. A data structure has been built around these AOPs, enabling them to be used as a framework to associate and contextualise different types of evidence (predictions and data), allowing for better safety assessment decisions to be made – read more about our approach; Beyond adverse outcome pathways: making toxicity predictions from event networks, SAR models, data and knowledge.
If you are interested in combining individual pieces of evidence in a meaningful way within your safety assessments, then please get in touch. We have a number of ongoing Lhasa-led industry conversations, which you may be interested in participating in, such as our ICH S1 consortium.
In the meantime, for an insight on how AOPs can be easily investigated as a network of interconnected key events, you may like to view Wiki Kaptis. Free application Wiki Kaptis – which has resulted from collaboration between Lhasa and an OECD working group – offers improved visualisation of public AOP-Wiki AOPs.
______
*An AOP is an approach to documenting causal relationships between biological processes which lead to adverse outcomes / toxicity. AOPs start with a molecular initiating event (MIE) and through additional key events (KEs), lead to an adverse outcome (AO). Each sequential KE (including the MIE and AO) are connected to each other through key event relationships (KERs). Each KE should be measurable and therefore can be linked to a relevant assay.
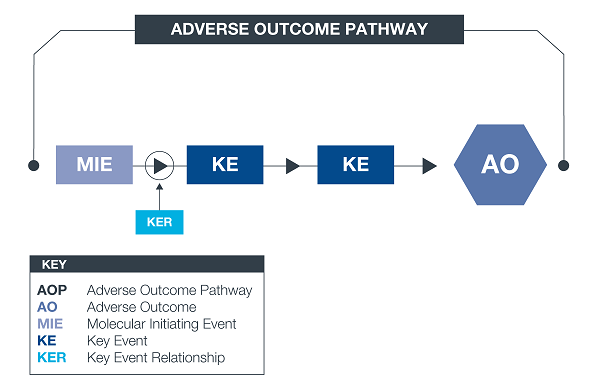 |
** An AOP network allows interconnecting KEs across different AOPs to be easily investigated as a network.
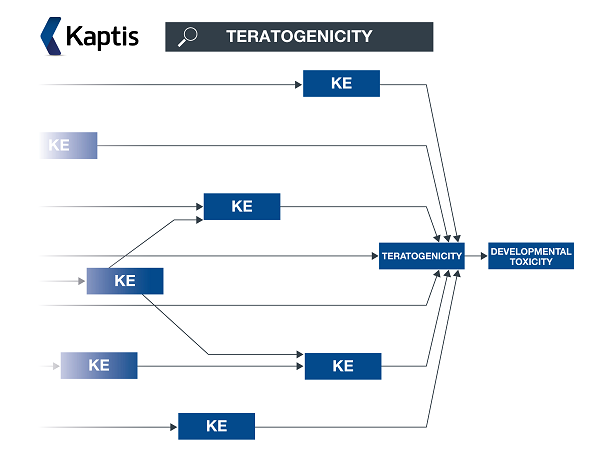 |
Last Updated on July 26, 2024 by lhasalimited



