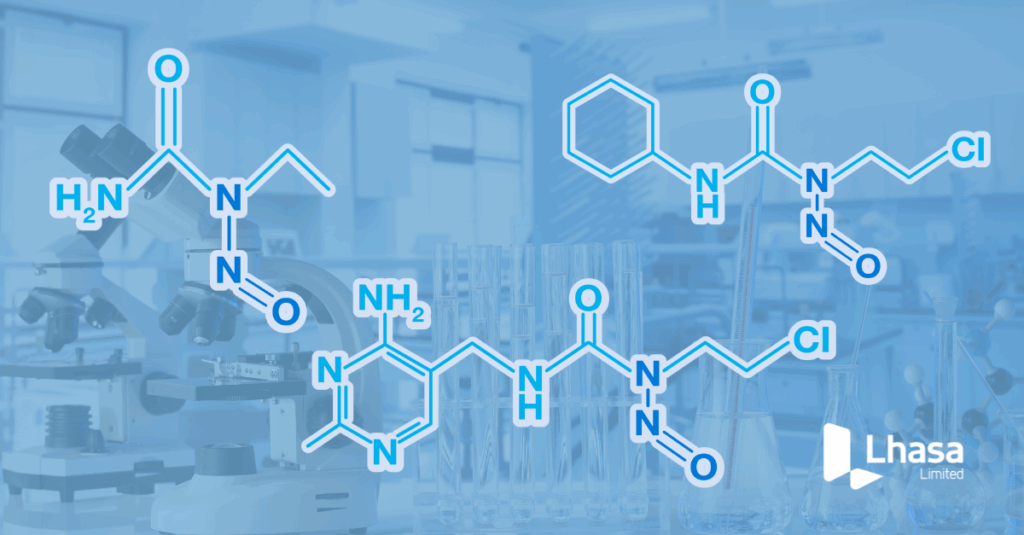
Navigating the EMA guidelines: Understanding nitrosourea acceptable intake limits via read-across methods
In the ever-evolving landscape of pharmaceutical regulations, staying abreast of the latest guidelines is crucial for ensuring the safety and efficacy of drug products. Recently, the European Medicines Agency (EMA) updated its guidelines to include specific nitrosourea acceptable intake (AI) limits – a class of N-nitroso compounds known for their potential carcinogenicity. In our recent […]