Q&A deep dive with Martin Walter: How to establish AI limits for NDSRIs
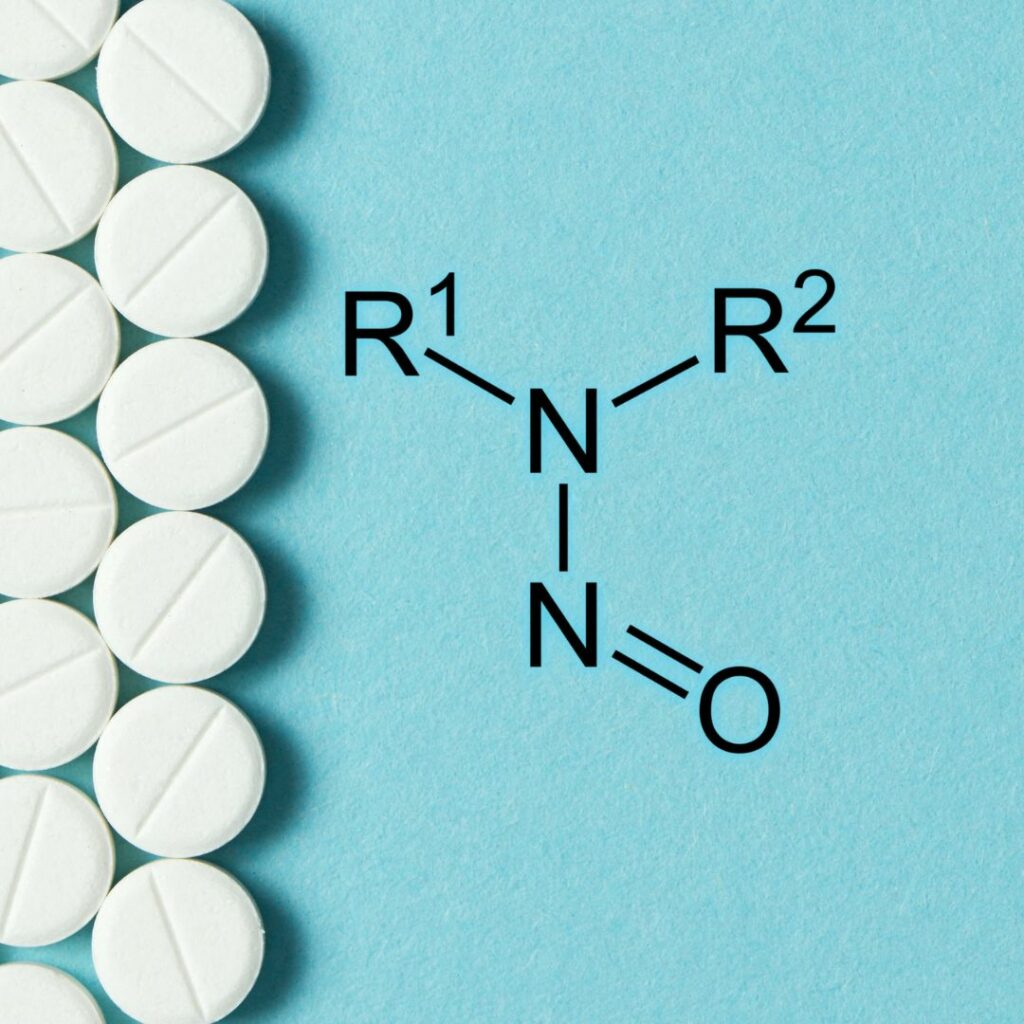
Building on the success of our recent webinar, How to establish acceptable intake (AI) limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs), we’re thrilled to present a follow-up that brings you closer to the insightful discussions that unfolded during the interactive Q&A session. In this blog, we’ve gathered questions posed during the webinar and curated written […]
Q&A deep dive with Martin Walter: How to establish AI limits for NDSRIs Read More »