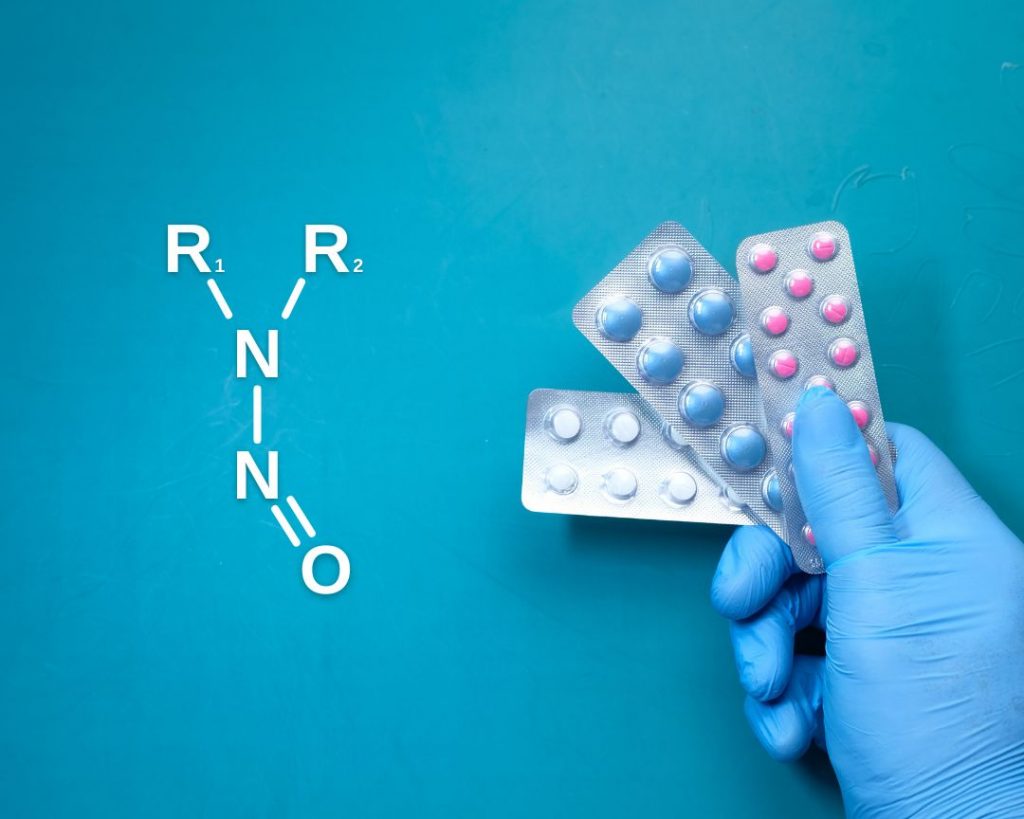
Supporting N-nitrosamine risk assessments for drug products
The nitrosamine crisis first erupted in July 2018, when the FDA announced voluntary recall of several drugs containing Valsartan: a medicine used to treat high blood pressure and heart failure. Due to an unreported change in synthesis of Valsartan, the potentially cancer-causing chemical N-nitrosodimethylamine (or NDMA) was detected in the marketed drug product. This resulted […]
Supporting N-nitrosamine risk assessments for drug products Read More »