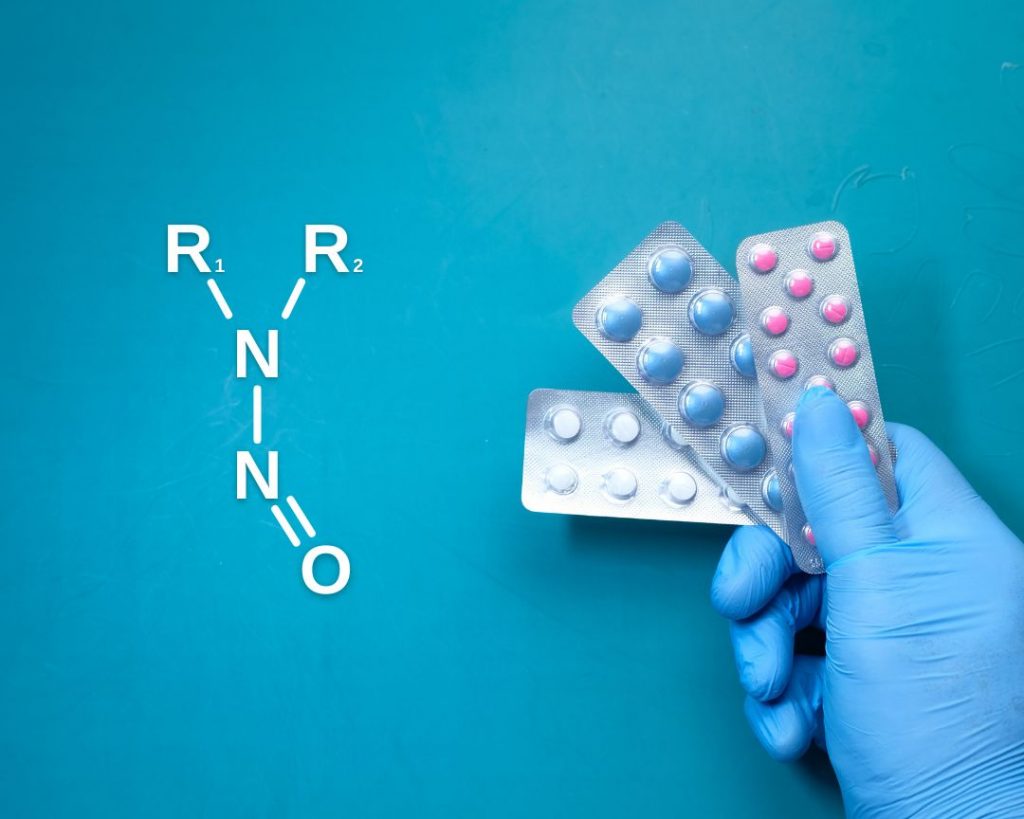
What does the EMA update on nitrosamine impurities mean for you?
Revised guidance released by the European Medicines Agency (EMA) on 7th July sparked excitement within the scientific community. By unveiling a new system to classify nitrosamines, EMA demonstrates that regulators are supporting industry in solving the complex problem of nitrosamine impurity assessment. The new approach will benefit industry, regulators and ultimately patients. What exactly are the […]
What does the EMA update on nitrosamine impurities mean for you? Read More »