In the ever-evolving landscape of pharmaceutical regulations, staying abreast of the latest guidelines is crucial for ensuring the safety and efficacy of drug products. Recently, the European Medicines Agency (EMA) updated its guidelines to include specific nitrosourea acceptable intake (AI) limits - a class of N-nitroso compounds known for their potential carcinogenicity.
In our recent webinar, experts Natan Segretti (CSO, Consult Lhasa) and David Ponting (Principal Scientist, Lhasa Ltd) explored these updates and discussed the methodologies for assessing these limits using read-across. This blog will summarise the key insights from the webinar and discuss the expected implications of these guideline changes:
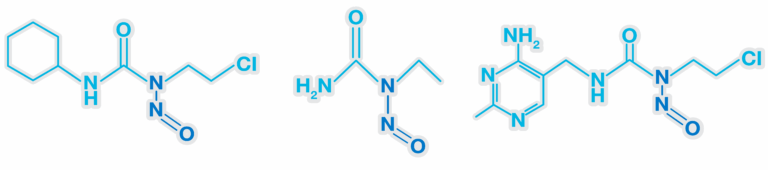
Understanding nitrosoureas and their risks
Nitrosoureas are part of a broader category of N-nitroso compounds, which have been a focal point in discussions about pharmaceutical impurities due to their potential to form highly reactive diazonium ions. These ions can alkylate DNA, potentially leading to mutations. Unlike nitrosamines, nitrosoureas do not require metabolic activation to exert their genotoxic effects, potentially making them a significant concern in risk assessments.
Natan commented on the challenges of these risk assessments:
"We have a lot of molecules that could represent a risk from the perspective of safe limits. Understanding why nitrosoureas and nitrosoguanidines could be a risk and should be looked at with more concern is crucial."
Natan Segretti
Nitrosoureas and the cohort of concern
To understand why nitrosoureas are worthy of their place in the cohort of concern, it is essential to delve into their mechanistic pathways, and the mechanisms through which they exert their genotoxic effects.
Nitrosoureas and compounds with similar functional groups such as guanidines and amides etc, can spontaneously form diazonium ions under physiological conditions. This formation does not require metabolic activation, differentiating them from nitrosamines, which rely on alpha hydroxylation for their carcinogenicity. This does however mean that the standard Ames test will be predictive for their mutagenic potential, and we would expect them to be mutagenic in the absence of S9.
David offered his view on the criteria for inclusion in the cohort of concern, and the nuances of assessing the potency of different compounds:
"I'm going to be a bit provocative here and say that we should be able to refine the cohort a bit here. The cohort is currently all N-nitroso compounds, but it was noted at the time that only about half of them have cohort-worthy potency. If it's got an alpha hydrogen for the metabolic activation, and it's a nitrosamine, or it's a nitrosated amide, urea, etc., then you can get to a diazonium ion. You have the risk of this extremely potent reaction and therefore it should be in the cohort.
On the other hand, if it is a nitrosated aromatic system, or any N-nitroso that doesn’t have two bonds to carbon, it’s difficult or impossible to form a diazonium ion and these shouldn’t be in the cohort. Nor should N-nitrosamines without alpha hydrogens, since these likewise have no route to a diazonium ion.”David Ponting
EMA guidelines on nitrosourea Acceptable Intake limits
The EMA’s updated guidelines set forth specific AI limits for certain nitrosoureas, reflecting their carcinogenic potency. For instance, the AI limit for several key guanidine-type nitrosoureas is established at 553 nanograms per day. These limits are derived from robust toxicity data, using the Carcinogenic Potency Categorisation Approach (CPCA) and read-across methods. The limits are designed to be protective, ensuring that patients are not exposed to levels of nitrosoureas that could pose significant health risks.
On the other hand, some aromatic N-nitroso compounds, which as discussed should not be in the cohort of concern, are considered safe at 1500 ng/day, the general TTC for mutagenic impurities – which these still potentially are, being mutagenic via a pathway comparable to aromatic amines.
On the other hand, some aromatic N-nitroso compounds—which should be excluded from the cohort of concern as previously discussed—are considered safe at the general Threshold of Toxicological Concern (TTC) of 1500 ng/day for mutagenic impurities. Despite not belonging to the cohort of concern, these compounds still maintain mutagenic potential through mechanisms similar to those of aromatic amines.
Read-across assessment of nitrosoureas
Read-across is a widely used method in toxicological risk assessment, allowing for the extrapolation of data from one substance to another based on structural and toxicological similarities.
In the webinar, David demonstrated the practical application of our in silico tool Acrostic via compound case studies, showing how the tool can be used to determine AI limits for nitrosoureas. Acrostic helps toxicologists to identify relevant analogues and assess their structural similarity, toxicological data, and pharmacokinetic properties to establish AI limits for their target compound.
The systematic read-across approach provided by Acrostic, ensures that the nitrosourea AI limits established are evidence-based and align with regulatory expectations.
Conclusion
The updated EMA guidelines and the use of read-across methods represent significant advancements in the risk assessment of nitrosoureas. By leveraging tools like Acrostic, pharmaceutical companies can ensure that their products meet safety standards and protect patients from potential carcinogenic risks. This webinar provided valuable insights into the regulatory framework and practical strategies for implementing these guidelines. To discuss Acrostic or any of Lhasa’s in silico tools, contact us here.
To request access to the webinar recording, please get in contact with the Lhasa marketing team.
Last Updated on April 16, 2025 by lhasalimited



