We had a fantastic time at EUROTOX 2024, held from 8 - 11th September in the picturesque city of Copenhagen, Denmark.
This year’s theme, “Toxicology – A Quest for Safe Chemicals and Medicines”, was reflected in the diverse sessions, posters and presentations throughout the event. Key topics ranged from the safety of drugs and environmental chemicals to emerging technologies, personalised medicine, and human health effects caused by exposure to chemicals.
We were thrilled to have a strong presence at the congress, where we actively engaged with the toxicology community through several key contributions:
An exhibitor-hosted session: Informing safety decisions with NAMs and AOPs using Kaptis
It was great to see a full room for our session, hosted by Senior Application Scientist, Toni Rogers and Principal Scientist, Alex Cayley. Their talk focused on leveraging new approach methodologies (NAMs) to optimise and accelerate safety assessments for carcinogenicity and other complex endpoints. If you missed the session and would like to access the slides, please email us at marketing@lhasalimited.org.
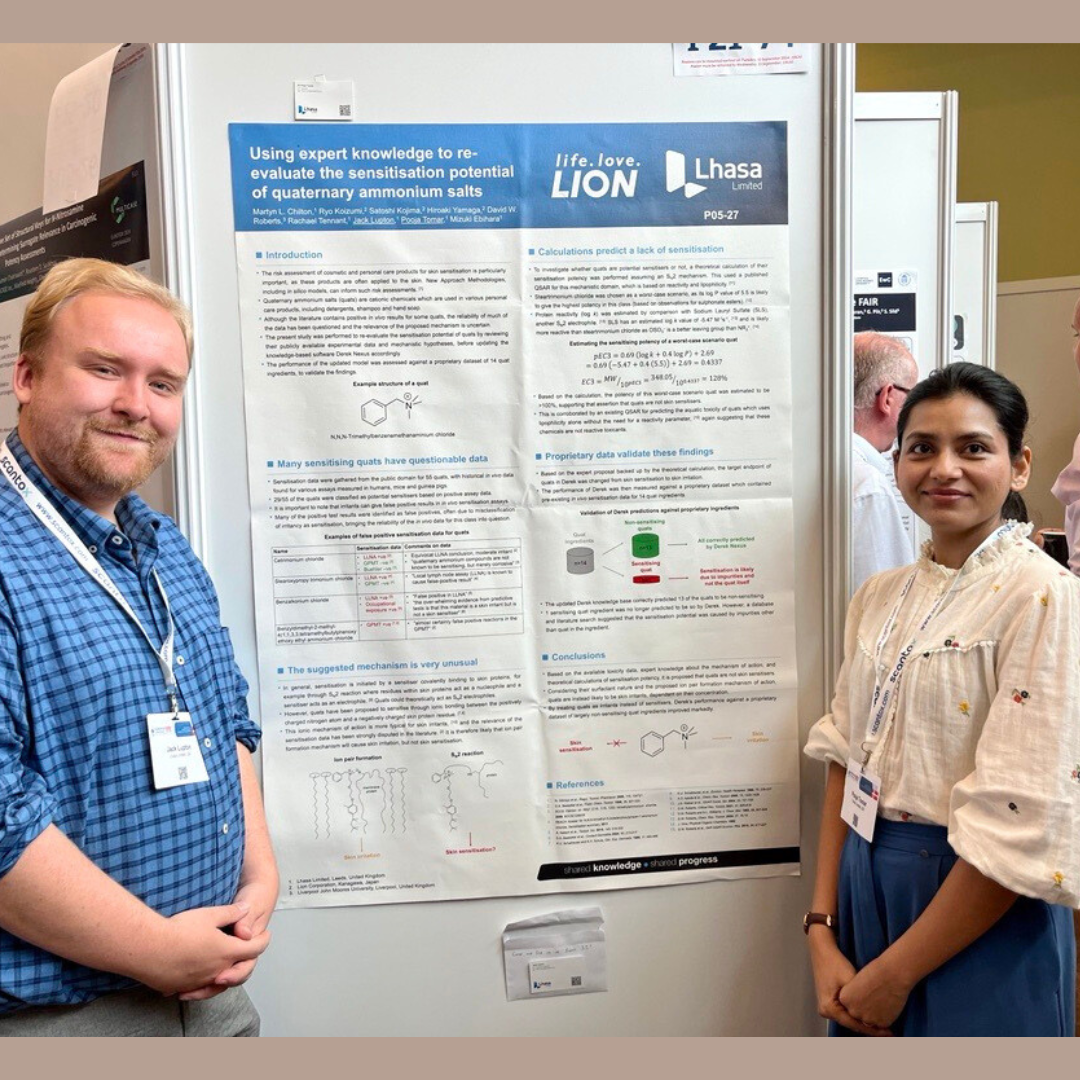
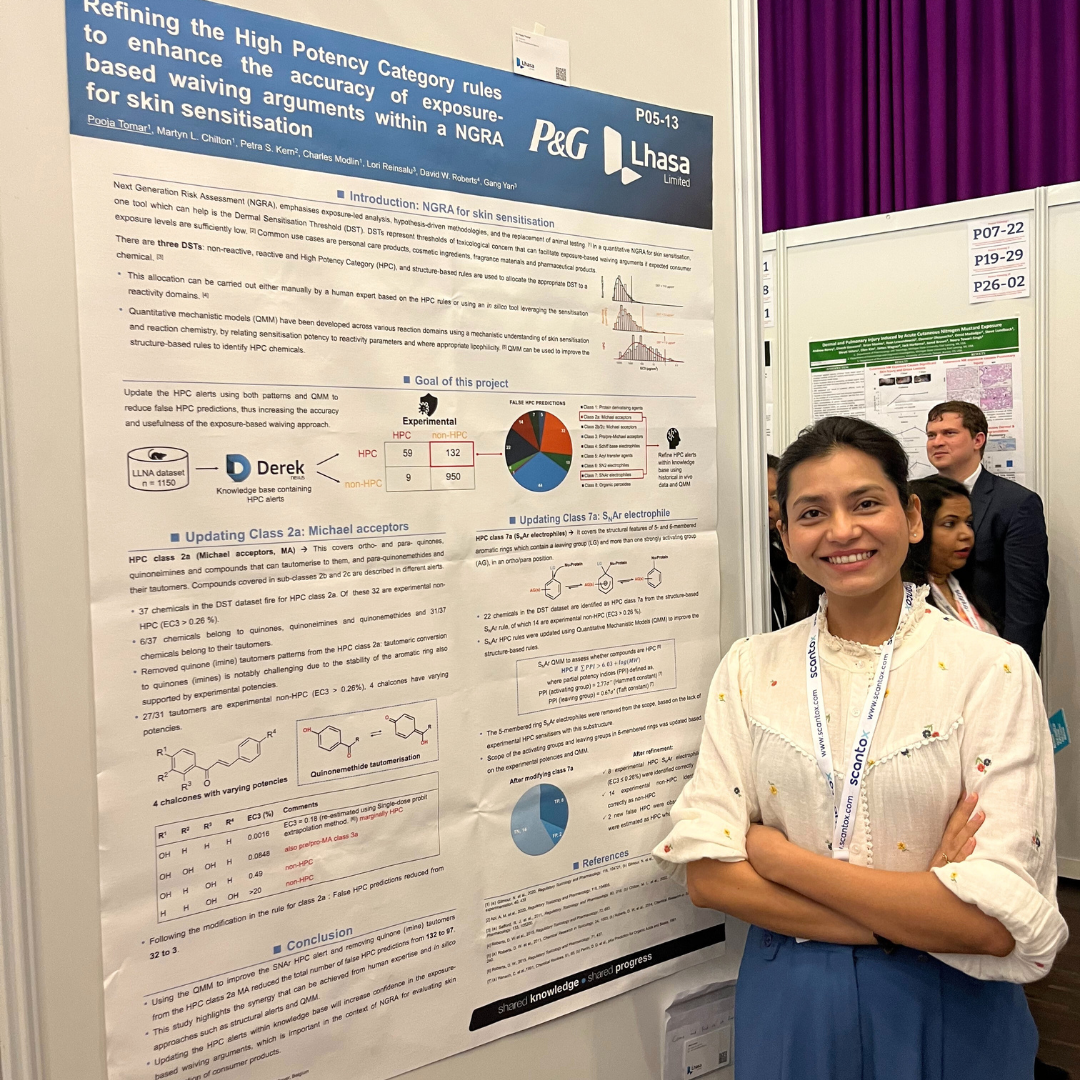
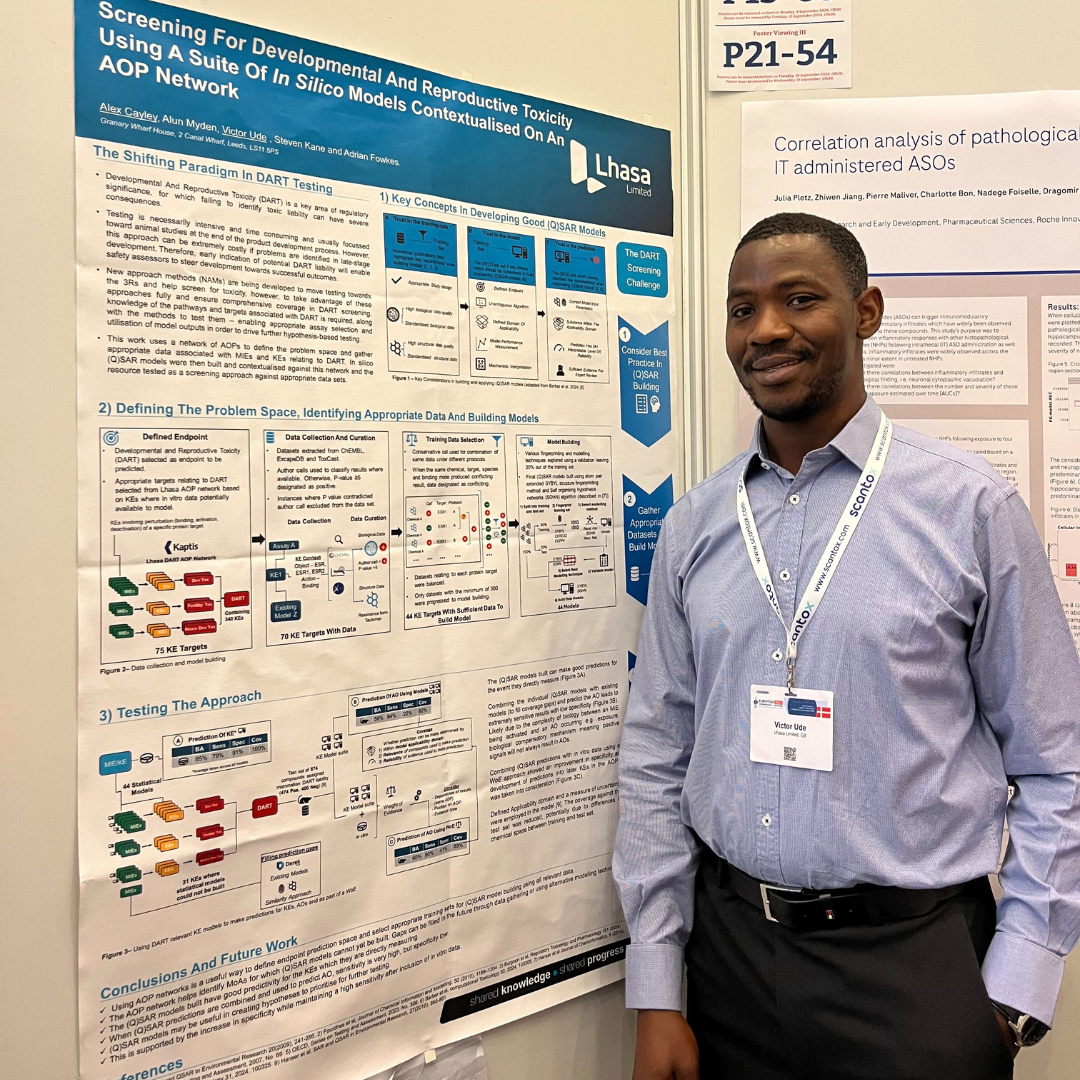
3 scientific posters and 1 short oral session
These sessions covered topics from developmental and reproductive toxicity (DART), next generation risk assessment (NGRA) for skin sensitisation, and contextualising NAMs on a network of adverse outcome pathways (AOPs). To receive PDF copies of our posters, contact us at marketing@lhasalimited.org.
A Lhasa booth (No. 33)
It was wonderful to connect with industry professionals and discuss how our in silico solutions can support various toxicological challenges and use cases.




Standout sessions: What caught our eye
In addition to our contributions, the Lhasa team attended a variety of scientific sessions.
This year’s programme was a powerful showcase of ongoing efforts to redefine risk assessment through NGRA. Industry progress in this area was evident, not only in scientific advancements, but also in the increasing acceptance of approaches that aim to minimise or eliminate the need for animal testing.
Many new technologies were presented, including:
- Omics-based screening methods for hazard identification.
- PBPK (Physiologically Based Pharmacokinetic) modelling to enable in vitro to in vivo extrapolation (IVIVE).
- And of course, in silico tools (including emerging AI technologies) which can access, organise and extend the knowledge base.
Two presentations were especially notable in the session titled “How to Build Trust in Artificial Intelligence for Toxicology?”. Dr Carsen Kneuer from the German Federal Institute for Risk Assessment (BfR) explored the motivation for integrating AI into toxicology and the challenges around building trust in these advanced technologies. This was followed up by philosopher, Dr Karim Jebari, who provided a thought-provoking perspective on the ethical dimensions of this approach. He emphasised the importance of transparency and accountability in decision-making – areas in which AI currently faces challenges. Dr Jebari argued that, while AI may struggle with replicating human judgment at higher levels, it could be invaluable in supporting more granular, data-driven decisions down the chain of command.
Another standout session was “Innovative In Vitro Screening Tools for Assessing the Risk of Immunotoxic Chemicals”, presented by Dr Dori Germolec from the National Institute of Environmental Health Sciences/NIH, Division of Translational Toxicology/NICEATM. This compelling presentation delved into the utility of in vitro platforms in detecting potential dermal sensitisers, stressing the importance of leveraging diverse methods and Defined Approaches (DAs) to enhance the predictive accuracy of skin sensitisation assessments. Dr Germolec’s talk highlighted the shift towards reducing dependence on animal testing while maintaining safety standards.
Let’s continue the conversation
Thank you to everyone who visited us at our booth, attended our exhibitor-hosted session, explored our scientific posters, and listened to our short oral presentation. We look forward to staying connected and continuing the conversation!
If you would like to receive a personalised demonstration of our in silico solutions, schedule a post Eurotox follow-up with a member of our business partnerships team.
Last Updated on November 12, 2024 by lhasalimited



