The saying goes, “It takes a village to raise a child”. This sentiment can be translated to the work we do at Lhasa Limited, particularly to the work which we put into building Adverse Outcome Pathways (AOPs). To echo the sentiment in Chris Barber’s previous blog post, The challenge of building QSAR models; it is easy to build an AOP, harder to build a useful AOP, and as for building an AOP you can trust? It takes a team.
Our Lhasa team have been developing AOPs, which lead to the carcinogenicity endpoint for the last couple of years, using knowledge derived from Derek Nexus and expert review (see figure 2 within the poster, Utilising Adverse Outcome Pathways as a Framework to Organise Evidence and Support Carcinogenicity Risk Assessment – also shown at the bottom of this article).
Our AOPs are captured in Kaptis, along with supporting information and evidence for each pathway. This expert, knowledge-based approach to building models is not a new concept to Lhasa, Derek Nexus alerts are made using the same principles, resulting in trusted, high-quality alerts. While it could potentially be quicker to put together AOPs using a more automated approach from a variety of sources, the approach we have taken offers the following benefits:
- The starting point for our AOPs comes from a high-quality and trusted source, Derek Nexus. Derek Nexus is our expert, knowledge-based toxicology software which gives predictions for a broad range of endpoints. Each alert is developed and peer-reviewed by a team of scientists. As well as predicting the toxicity of a compound, each alert details data, scope and proposed toxicity modes of action based on public and private data, and literature review. Therefore, the high-quality of the work within the software enables us to easily and confidently extract molecular initiating events (MIEs) and key events (KEs) relating to carcinogenicity.
- Each pathway is expert-reviewed, expanded or refined, and peer-reviewed. This process results in evidence supported AOPs which are well documented for the user, transparent and robust. Through our research, we know that there is significant detail to each pathway, which could be missed if using an automated approach.
AOPs are going to be of great importance in the future of safety toxicology. The need for alternatives to animal testing is of paramount importance in multiple industries across the world. The OECD recently published a guidance document which outlines the emerging alternative methods to toxicity testing and the need to use evidence from these approaches in an IATA. This OECD document suggests that AOPs could be applied as a framework to capture, organise and reason to come to a decision in a meaningful way. Therefore, having robust AOPs, built by a team of experts will give the scientific community the confidence to use this approach to make reasoned, transparent and defendable decisions.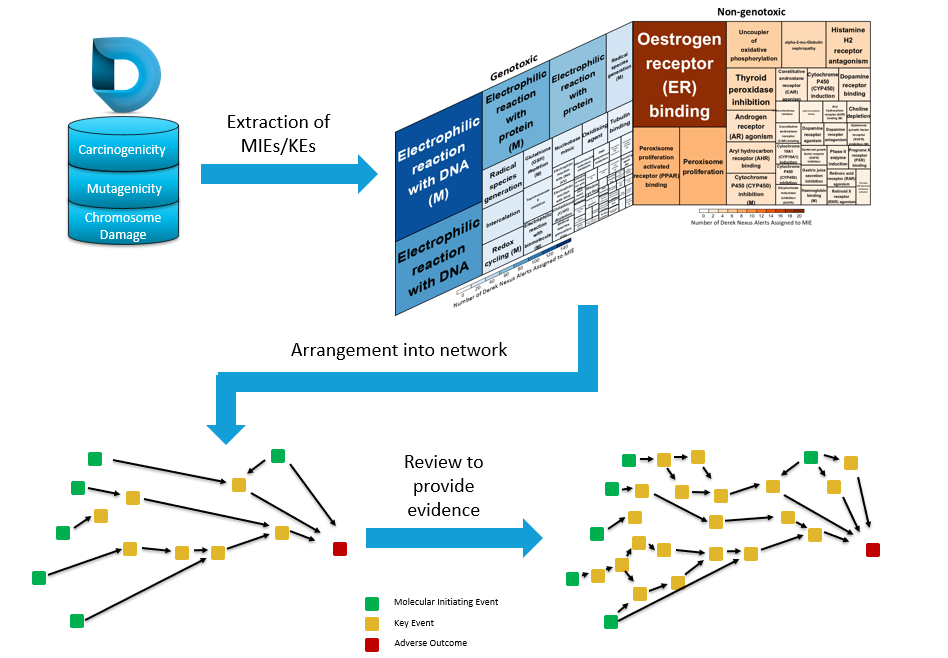
Approach to building an AOP framework, based on knowledge stored within Derek Nexus.
Last Updated on January 25, 2024 by lhasalimited