The introduction of purge arguments by Teasdale et al. has had a huge impact on the way in which companies consider the quality of their drug substances with respect to potentially mutagenic impurities (PMIs), allowing chemists to utilise their inherent knowledge of the physicochemical properties of the impurities within the synthetic process.1 This encourages a quality by design (QbD) approach, where the impurities are considered throughout the synthesis and the incorporation of additional processes to aid PMI removal, as opposed to simply analysing the final product – quality by testing (QbT).
Key to the regulatory acceptance of purge arguments is the conservatism within the purge factor scoring system, which for reactivity purge has been demonstrated across a number of publications and is consistently implemented across the pharmaceutical industry through the development of a collective understanding of when and how they should be applied.2–4 Likewise, volatility purge scoring is relatively simple to understand and apply, particularly using the scoring thresholds originally proposed by Teasdale et al. However, through our interactions with Mirabilis users, regulators, and other members of the purge community, it has become increasingly apparent that the same cannot be said of solubility purge scoring. This is perhaps understandable given the number of methods by which crude reaction mixtures can be processed to afford the clean intermediate/API.

Table 1. Theoretical Purge Factor Scoring System (click to view a larger version)
Lhasa have therefore identified the huge value to the community of developing an accepted best practice, incorporating both the application of solubility purge scoring and, where appropriate, provision of a clear justification of the purge factor assigned. As such, we have embarked on the process through engagement with Mirabilis users, and through discussion with multiple regulatory agencies, with the aim of developing a unified approach to the application of solubility purge scoring.
The key topics which are currently being addressed include:
Terminology. There are many terms that are used across work up and purification processes and it is important that when a term is utilised, the implication of the term is understood by everyone involved, and is utilised consistently throughout industry. In some instances, a named process may carry with it an implication of another process, e.g. a crystallisation would generally infer a method of separating the solids from the mother liquors (see figure 1 below).
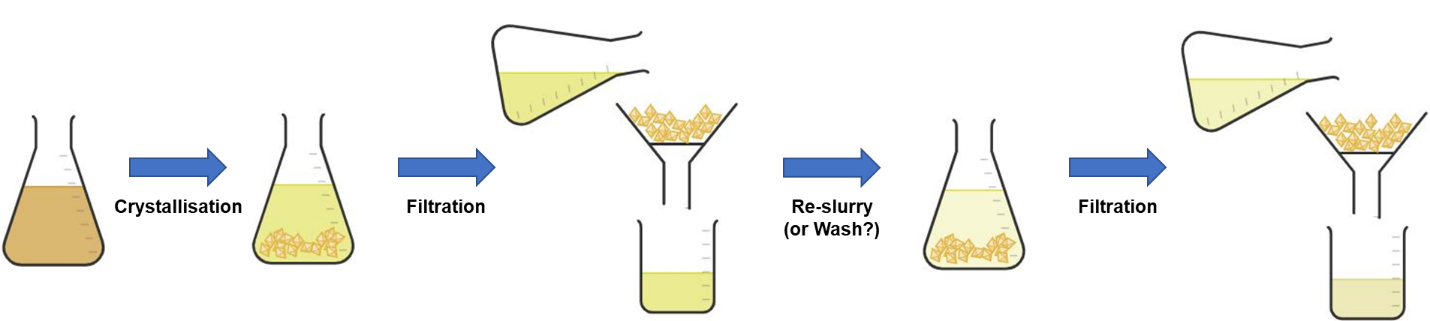
Figure 1: Crystallisation – One process with four implicit operations, or multiple separate processes?
Application. The application of purge factors associated with solubility are currently handled very differently across the industry and therefore offer an increased possibility to be misapplied. The effect of misapplication of solubility purge factors could have the unintentional effect of endangering the conservatism of the approach. This can, in part, be linked to different interpretations of the terminology, however outside of this confusion can arise from whether repeat processes, such as extractions, can be scored multiple times. (Figure Y)
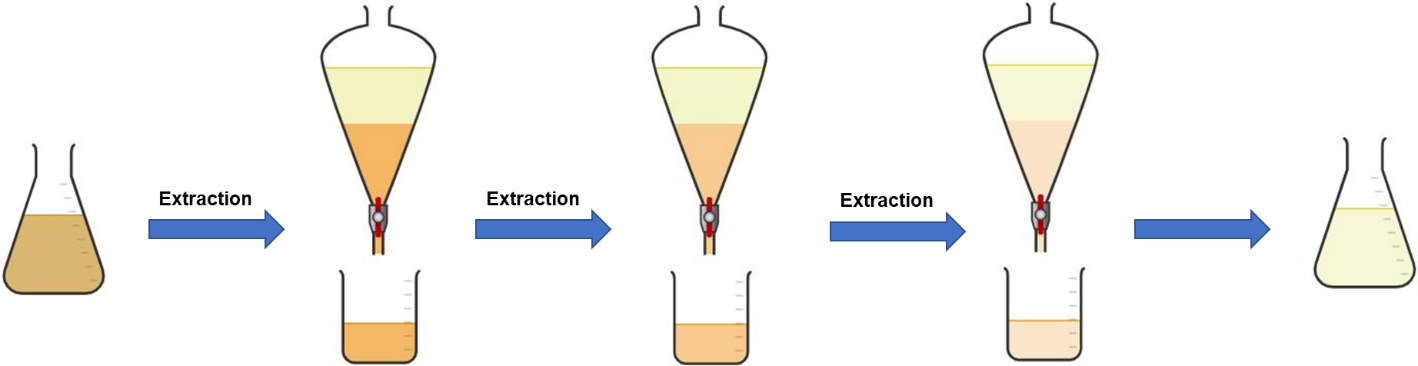
Figure 2: Extraction – One process or three? One purge or three?
Justification. Clear guidance towards when and how a suitable justification should be provided for solubility purge factors would aid preparation of regulatory filings; indicating where data collection or a prediction may be necessary. This type of framework, like those previously developed within the Mirabilis consortium5, would lead to a clear expectation within submissions and pre-empt likely questions which could arise during regulatory review.
To date, work has focused on the appropriate implementation of solubility purge factors relating to liquid-liquid extractions and identifying where provision of experimental evidence is necessary and where suitable predictive methods could support the purge. A necessary part of incorporating the use of predictions into the best practice guidance will involve experimental data to support the validity and conservatism of the predictions, with members of the group also looking to identify where they can contribute relevant publishable data to aid any validation.
The ultimate goal of this exercise is to publish a best practice approach that can be easily utilised in our members workflows, through incorporation directly into Mirabilis, but also the wider purge community across the pharmaceutical industry. By defining a clear format for the application, disclosure and provision of supporting evidence, submissions utilising the purge approach will become more consistent and require less back and forth between industry and regulators, thereby increasing engagement with the approach. The outcome will hopefully facilitate quicker risk assessments, submissions and regulatory acceptance, and ultimately speed up drug availability to patients.
References
[1] Teasdale, A.; Fenner, S.; Ray, A.; Ford, A.; Phillips, A. A Tool for the Semiquantitative Assessment of Potentially Genotoxic Impurity (PGI) Carryover into API Using Physicochemical Parameters and Process Conditions. Org. Process Res. Dev. 2010, 14 (4), 943–945. https://doi.org/10.1021/op100071n.
[2] Burns, M. J.; Ott, M. A.; Teasdale, A.; Stalford, S. A.; Antonucci, V.; Baumann, J.-C.; Brown, R.; Covey-Crump, E. M.; Elder, D.; Elliott, E.; et al. A New Semi-Automated Computer-Based System for Assessing the Purge of Mutagenic Impurities. Org. Process Res. Dev. 2019, 23 (11). https://doi.org/10.1021/acs.oprd.9b00358.
[3] Urquhart, M. W. J.; Bardsley, B.; Edwards, A. J.; Giddings, A.; Griva, E.; Harvey, J.; Hermitage, S.; King, F.; Leach, S.; Lesurf, C.; et al. Managing Emerging Mutagenicity Risks: Late Stage Mutagenic Impurity Control within the Atovaquone Second Generation Synthesis. Regul. Toxicol. Pharmacol. 2018, 99, 22–32. https://doi.org/https://doi.org/10.1016/j.yrtph.2018.08.004.
[4] Betori, R. C.; Kallemeyn, J. M.; Welch, D. S. A Kinetics-Based Approach for the Assignment of Reactivity Purge Factors. Org. Process Res. Dev. 2015, 19 (11), 1517–1523. https://doi.org/10.1021/acs.oprd.5b00257.
[5] Barber, C.; Antonucci, V.; Baumann, J.-C.; Brown, R.; Covey-Crump, E.; Elder, D.; Elliott, E.; Fennell, J. W.; Gallou, F.; Ide, N. D.; et al. A Consortium-Driven Framework to Guide the Implementation of ICH M7 Option 4 Control Strategies. Regul. Toxicol. Pharmacol. 2017, 90, 22–28. https://doi.org/10.1016/J.YRTPH.2017.08.008.
Last Updated on January 25, 2024 by lhasalimited



