Ensuring the safety and efficacy of active pharmaceutical ingredients (APIs) is of paramount importance. A critical aspect of this process involves the identification and mitigation of potentially mutagenic impurities, like nitrosamines. In this blog, we will explore the significance of risk mitigation at the early stages of API development and introduce a cutting-edge solution, Zeneth, that can help in understanding degradation pathways, including those leading to nitrosamine formation.
Zeneth is the solution for scientists who need to understand the forced degradation pathways of organic compounds. One prominent use case of Zeneth is within exploratory formulations departments, to understand and account for any potential degradation problems within the API at an early stage. This advanced understanding informs decisions around experiment and excipient selection, as well as identifying any testing requirements.
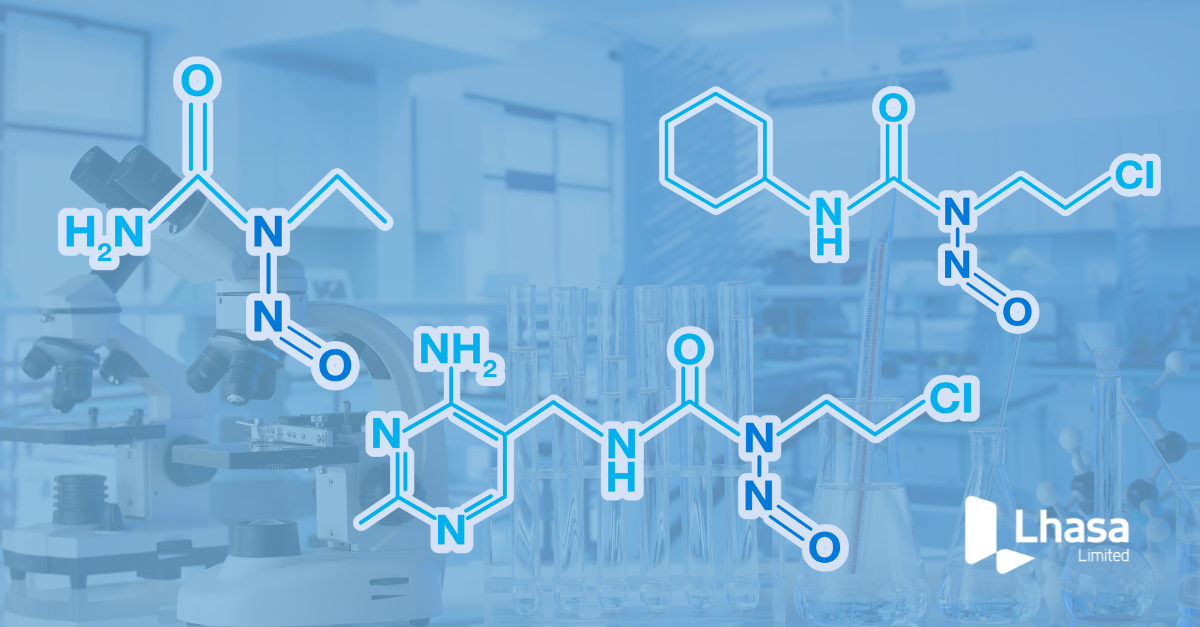
Prediction of pathways that lead to nitrosamine degradant formation has been greatly expanded in Zeneth, as a result of nitrites present as excipients, or impurities in excipients under certain conditions. More complex routes to nitrosamines where reliance on several different environmental conditions to be predicted have also been considered. The addition of this chemistry has allowed Zeneth to assess the formation of nitrosamines as degradants for a range of nitrogen containing compounds under the specific reaction conditions.
Rodrigo Silva, Analytical research and development manager from Santisa, a Lhasa member that sponsors Zeneth, shares his experience, “Zeneth has improved the prediction of interactions between drugs and excipients, ensuring a more efficient and safer formulation. With completely reliable results provided by Zeneth, the quality of our work has increased considerably, boosting productivity and confidence in daily activities.”
How can Zeneth help with risk mitigation?
- Degradant Prediction: Zeneth can swiftly provide predictions for the likely degradation products of the API whilst considering the effect of excipients and their impurities within the final formulated product. It can anticipate potential impurities, including nitrosamines, that may form under different conditions.
- Degradant Identification: Zeneth has comprehensive knowledge of degradation pathways which ensures structure elucidation of experimentally observed degradants. Intuitive tools allow filtering of results based on chemical formula, mass and mass difference allowing experimental data to guide predictions.
- Risk Assessment: Zeneth provides tools for assessing the risks associated with the identified degradation pathways. This enables pharmaceutical companies to prioritize and address the most critical issues early in the development process.
- Regulatory Compliance: Numerous guidelines (ICH Q1A, ICH Q1B, ICH Q3A, ICH Q3B, ICH M7 and RDC 53) stipulate the need to understand the consequences of degradation and focus on impurities and stability testing. The decision support that Zeneth offers can be invaluable in meeting many of these requirements. Under ICH M7, potential degradation products likely to be present in the final drug product should be evaluated for mutagenic potential.
In the world of pharmaceutical development, early risk mitigation is fundamental for ensuring the safety and efficacy of APIs. The formation of impurities, such as nitrosamines, can have serious consequences for patient health, regulatory compliance, and a company’s reputation. Zeneth offers a cutting-edge solution to understand degradation pathways and predict the formation of impurities, thereby empowering pharmaceutical companies to take proactive steps in risk mitigation. Find out more about our solutions for impurity and degradant control and nitrosamine risk assessment. If you would like more information, get in touch.
Last Updated on January 25, 2024 by lhasalimited