Have you read our collaborative paper, published with the National Institute of Health Sciences (NIHS) of Japan? The paper is based on our shared project to improve in silico developmental and reproductive toxicity (DART) predictions, to safely move towards replacing traditional animal testing for chemical safety assessments.
“The outcome of this collaborative and innovation project is an important and exciting move towards improved safety assessments, which incorporate alternative testing”– Dr Takashi Yamada, Section Chief, Division of Risk Assessment, Center for Biological Safety and Research at NIHS of Japan.
NIHS
The National Institute of Health Sciences (NIHS) conducts testing, research, and studies toward the proper evaluation of the quality, safety, and efficacy of pharmaceutical products, foods, and the numerous chemicals in the living environment.
Reducing animal testing
The global drive to reduce, refine and replace animal testing for human safety assessments is now evident across several industries:
- Progress has been made within the cosmetics industry in relation skin sensitisation, where defined approaches have been created – initiated by the 2013 European Union ban on testing of new cosmetic products on animals.
- In 2021, the US Environmental Protection Agency (US EPA) pledged to eliminate animal testing by 2035.
- In 2022, the Food and Drug Administration (FDA) shared plans to reform the drug approval process and to drive the use of non-animal testing methods and streamline potential life-saving medication for patients.
New approach methodologies (NAMs) and integrated approaches to testing and assessments (IATAs) are being developed to improve the human relevance of safety assessments and reduce the need for animal testing. NAMs and IATAs can be framed around adverse outcome pathways (AOPs)* which improve the mechanistic understanding of, and increase the confidence in, the use of animal alternatives. A broad coverage of AOPs for each endpoint, increases the confidence in this approach further.
We have previously demonstrated how AOPs can be used to contextualise a variety of (Q)SAR approaches, leading to improved risk assessment strategies during compound prioritisation and labelling. This work has been selected as the best paper published in Reproductive Toxicology in 2022.
The collaborative project
In this collaborative project – between the NIHS of Japan and Lhasa – we examined the performance of a predictive model based on a developmental and reproductive toxicity (DART) AOP network created by Lhasa, against a novel NIHS toxicity dataset. The NIHS dataset contains studies performed according to OECD test guidelines.
The aim of the project was to test and improve the DART AOP knowledge, and has resulted in the development of several DART-related AOPs.
This work builds on our other Lhasa AOP work to date – for the carcinogenicity endpoint this knowledge is now accessible within in silico software, Kaptis – which is being used to improve carcinogenicity assessment.
Read the open access paper – Enhancing developmental and reproductive toxicity knowledge: A new AOP stemming from glutathione depletion – for more information on:
- Our successful collaboration with the NIHS of Japan.
- The progress made towards better predictive DART models for improved safety assessment.
- Full details of one of the new AOPs created for male fertility toxicity.
If you would like more information, please get in touch.
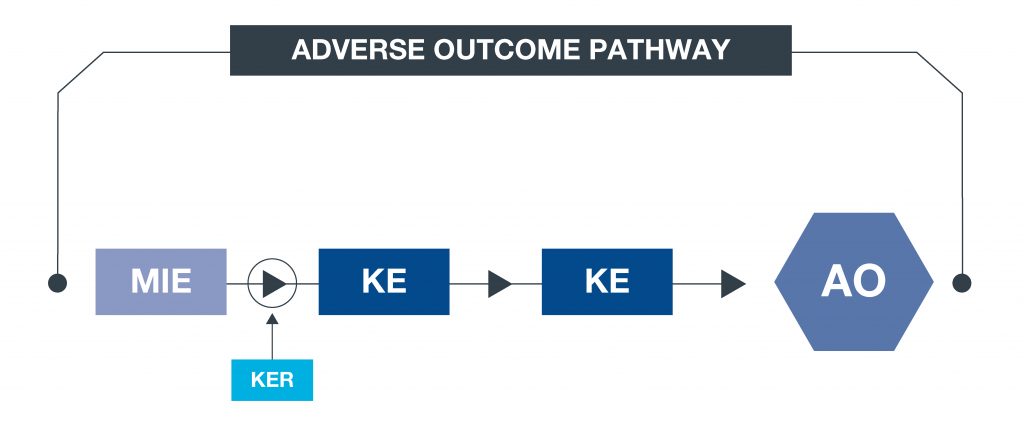
*What is an AOP? An approach to documenting causal relationships between biological processes which lead to adverse outcomes/toxicity. AOPs start with a molecular initiating event (MIE) and through additional key events (KEs), lead to an adverse outcome (AO). Each sequential KE (including the MIE and AO) are connected to each other through key event relationships (KERs). Each KE should be measurable and therefore can be linked to a relevant assay. This framework enables data to be contextualised and organised in an intuitive way, to support decisions with respect to chemical safety assessments.
Last Updated on January 25, 2024 by lhasalimited